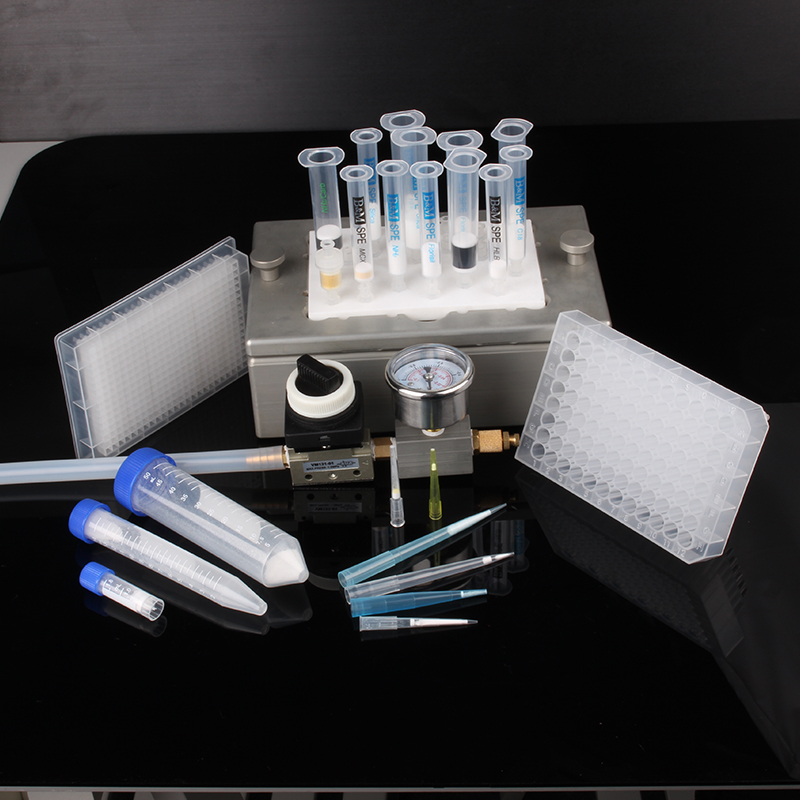
Solid phase extraction is a sample pretreatment technology developed in recent years. It is developed from the combination of liquid-solid extraction and column liquid chromatography. It is mainly used for sample separation, purification and concentration. Compared with traditional liquid-liquid extraction Improve the recovery rate of the analyte, separate the analyte from the interfering components more effectively, reduce the sample pretreatment process, and the operation is simple, time-saving and labor-saving. It is widely used in medicine, food, environment, commodity inspection, chemical industry and other fields.
Extraction is a unit operation that uses the different solubility of the components in the system to separate the mixture. There are two ways to extract:
Liquid-liquid extraction, a selected solvent is used to separate a certain component in a liquid mixture. The solvent must be immiscible with the extracted mixture liquid, have selective solubility, and must have good thermal and chemical stability, and There is little toxicity and corrosiveness. Such as separation of phenol with benzene; separation of olefins in petroleum fractions with organic solvents.
Solid phase extraction, also called leaching, uses solvents to separate the components in the solid mixture, such as leaching sugars in sugar beets with water; leaching soybean oil from soybeans with alcohol to increase oil yield; leaching active ingredients from traditional Chinese medicine with water The preparation of liquid extract is called “leaching” or “leaching”.
Although extraction is often used in chemical experiments, its operation process does not cause changes in the chemical composition of the extracted substances (or chemical reactions), so the extraction operation is a physical process.
Extractive distillation is distillation in the presence of an easily soluble, high boiling point, and non-volatile component, and this solvent itself does not form a constant boiling point with other components in the mixture. Extractive distillation is usually used to separate some systems with very low or even equal relative volatility. Since the volatility of the two components in the mixture are nearly equal, the solid phase extractor makes them evaporate at nearly the same temperature, and the degree of evaporation is similar, making separation difficult. Therefore, relatively low volatility systems are usually difficult to separate by a simple distillation process.
Extractive distillation uses a generally non-volatile, high boiling point, and easily soluble solvent to mix with the mixture, but does not form a constant boiling point with the components in the mixture. This solvent interacts with the components in the mixture differently, causing their relative volatility to change. So that they can be separated during the distillation process. The highly volatile components are separated and form the overhead product. The bottom product is a mixture of solvent and another component. Since the solvent does not form an azeotrope with another component, they can be separated by a suitable method.
An important part of this distillation method is the choice of solvent. The solvent plays an important role in separating the two components. It is worth noting that when choosing a solvent, the solvent needs to be able to significantly change the relative volatility, otherwise it will be a futile attempt. At the same time, pay attention to the economics of the solvent (the amount that needs to be used, its own price and its availability). It is also easy to separate in the tower kettle. And it can’t chemically react with each component or mixture; it can’t cause corrosion in the equipment. A typical example is the use of aniline or other suitable substitutes as a solvent to extract the azeotrope formed by distilling benzene and cyclohexane.
Solid phase extraction is a widely used and increasingly popular sample pretreatment technology. It is based on traditional liquid-liquid extraction and combines the similar dissolution mechanism of substance interaction with the widely used HPLC and GC. The basic knowledge of stationary phases in the book gradually developed. SPE has the characteristics of small amount of organic solvents, convenience, safety, and high efficiency. SPE can be divided into four types according to its similar dissolution mechanism: reverse phase SPE, normal phase SPE, ion exchange SPE, and adsorption SPE.
SPE is mostly used to process liquid samples, extracting, concentrating and purifying semi-volatile and non-volatile compounds in them. It can also be used for solid samples, but must be processed into liquid first. At present, the main applications in China are the analysis of organic substances such as polycyclic aromatic hydrocarbons and PCBs in water, the analysis of pesticide and herbicide residues in fruits, vegetables and food, the analysis of antibiotics, and the analysis of clinical drugs.
The SPE device is composed of an SPE small column and accessories. SPE small column is composed of three parts, column tube, sintered pad and packing. SPE accessories generally include a vacuum system, a vacuum pump, a drying device, an inert gas source, a large-capacity sampler and a buffer bottle.
A sample including separated substances and interferences passes through the adsorbent; the adsorbent selectively retains the separated substances and some interferences, and other interferences pass through the adsorbent; rinse the adsorbent with a suitable solvent to make the previously retained interferences selective After leaching off, the separated material remains on the adsorbent bed; the purified and concentrated separated material is washed from the adsorbent.
Solid phase extraction is a physical extraction process that includes liquid and solid phases. In solid phase extraction, the adsorption force of solid phase extractor against the separation is greater than that of the solvent that dissolves the separation. When the sample solution passes through the adsorbent bed, the separated substance is concentrated on its surface, and other sample components pass through the adsorbent bed; through the adsorbent that only adsorbs the separated substance and does not adsorb other sample components, a high-purity and concentrated separator can be obtained.
Post time: Mar-09-2021